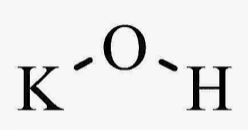
Is KOH an Electrolyte? Exploring the Role of Potassium Hydroxide in Electrolyte Solutions
Share
In the realm of chemistry and electrolyte solutions, a common question is, "Is KOH (Potassium Hydroxide) an electrolyte?" Understanding the nature of KOH and its role as an electrolyte is crucial for both scientific and practical applications. In this blog post, we'll delve into the properties of potassium hydroxide and its classification as an electrolyte.
What is Potassium Hydroxide (KOH)?
Potassium hydroxide, commonly known as KOH, is an inorganic compound with a strong alkaline nature. It's a white solid, often found in pellet or flake form, and is highly soluble in water, producing a heat-releasing reaction.
Chemical Properties of KOH
- Strong Base: KOH is a strong base, meaning it can accept protons (H+) in reactions.
- Hygroscopic: It readily absorbs moisture from the air, making it an efficient drying agent.
- Reactivity: KOH reacts with acids to form salts and water, a typical acid-base neutralization reaction.
Is KOH an Electrolyte?
Yes, KOH is classified as an electrolyte. Here’s why:
- Ionic Dissolution: When dissolved in water, KOH dissociates into potassium (K+) ions and hydroxide (OH-) ions. This dissociation is a key characteristic of electrolytes.
- Conductivity: The presence of free-moving ions (K+ and OH-) in KOH solutions allows it to conduct electricity, another hallmark of electrolyte solutions.
- Use in Electrolyte Solutions: KOH is often used in various applications where electrolyte solutions are required, such as in batteries and biodiesel production.
The Role of KOH in Electrolyte Solutions
In electrolyte solutions, KOH serves several functions:
- Battery Electrolyte: KOH is used in alkaline batteries, where it acts as the electrolyte, facilitating the flow of ions and, consequently, electricity.
- Chemical Reactions: In laboratories and industry, KOH solutions are used in chemical synthesis and analysis, benefiting from its properties as a strong base and electrolyte.
Safety and Handling
While KOH is a valuable chemical in various applications, it's important to handle it with care:
- Corrosive Nature: KOH can cause chemical burns and is corrosive to some metals.
- Safety Precautions: Always use protective gear, such as gloves and goggles, when handling KOH. Work in a well-ventilated area and follow all safety guidelines.
Conclusion
Potassium hydroxide (KOH) is indeed an electrolyte, with properties that make it valuable in various scientific and industrial applications. Its ability to dissociate into ions and conduct electricity defines its role as an electrolyte. However, its handling requires careful safety measures due to its corrosive nature.
For more information on electrolytes and their applications, visit drinksote.com. Discover the fascinating world of electrolytes and their significance in various fields, from chemistry to health and beyond.

